Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Si
Si O
O
Suzuki, Eriko; Nakajima, Kunihisa; Osaka, Masahiko; Oishi, Yuji*; Muta, Hiroaki*; Kurosaki, Ken*
Journal of Nuclear Science and Technology, 57(7), p.852 - 857, 2020/07
Times Cited Count:4 Percentile:44.4(Nuclear Science & Technology)The low temperature heat capacity of Cs Si
Si O
O , which is one of the cesium chemisorbed compounds onto stainless steel during severe accident of the light water nuclear reactor, was experimentally determined for the first time in the temperature range of 1.9 - 302 K. The experimentally determined heat capacity,
, which is one of the cesium chemisorbed compounds onto stainless steel during severe accident of the light water nuclear reactor, was experimentally determined for the first time in the temperature range of 1.9 - 302 K. The experimentally determined heat capacity, 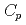
 (298.15K), and the standard entropy,
(298.15K), and the standard entropy, 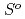 (298.15K), were 249.4
(298.15K), were 249.4  1.1 J K
1.1 J K mol
mol and 322.1
and 322.1  1.3 J K
1.3 J K mol
mol , respectively. The standard Gibbs energy of formation of Cs
, respectively. The standard Gibbs energy of formation of Cs Si
Si O
O at high temperatures,
at high temperatures, 

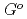 (
( ), were reevaluated by using the presently obtained
), were reevaluated by using the presently obtained 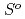 (298.15K) and the previously reported experimental results of the standard enthalpy of formation,
(298.15K) and the previously reported experimental results of the standard enthalpy of formation, 

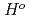 (298.15K), and the standard enthalpy increments at high temperatures,
(298.15K), and the standard enthalpy increments at high temperatures, 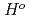 (
( )-
)-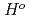 (298.15K).
(298.15K).
Kobayashi, Yoshinao*; Ueda, Shigeru*; Sato, Isamu*; Osaka, Masahiko
no journal, ,
SiO and Si(OH) can be Si source for Cs-bearing particle and other in-reactor compounds during Fukushima-Daiichi NPS accident. Dependencies of vapor pressure of those species on oxygen partial pressure and tenperature were evaluated thermodunamically. And experimental condition by chemical equilibrium method was determined in focusing Cs
can be Si source for Cs-bearing particle and other in-reactor compounds during Fukushima-Daiichi NPS accident. Dependencies of vapor pressure of those species on oxygen partial pressure and tenperature were evaluated thermodunamically. And experimental condition by chemical equilibrium method was determined in focusing Cs O-SiO
O-SiO system.
system.
Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Oishi, Yuji*; Muta, Hiroaki*; Kurosaki, Ken*
no journal, ,
Cesium-silicates were formed by Cs chemisorption onto stainless steel under LWR severe accident condition. To investigate thermodynamic property of the cesium-silicates, low temperature heat capacities of the cesium-silicates were measured. The heat capacity and entropy of Cs Si
Si O
O at 298K calculated by measurement data generally corresponded with estimated data by Neumann-Kopp's rule and previous report.
at 298K calculated by measurement data generally corresponded with estimated data by Neumann-Kopp's rule and previous report.